SL Paper 1
Which solution forms when phosphorus(V) oxide, P4O10, reacts with water?
Which property increases down Group 1, the alkali metals?
A. Atomic radius
B. Electronegativity
C. First ionization energy
D. Melting point
Which trend is correct, going down group 1?
A. Melting point increases
B. Reactivity decreases
C. First ionisation energy increases
D. Electronegativity decreases
Which describes an atom of bismuth, Bi (Z = 83)?
Which is a d-block element?
A. Ca
B. Cf
C. C
D. Co
Which of the following would have the same numerical value for all elements in the same period?
A. Highest energy levels occupied
B. Energy sub-levels occupied
C. Orbitals occupied
D. Valence electrons
Which species has the same electron configuration as argon?
A. Br−
B. Ca2+
C. Al3+
D. Si4+
Which oxide will dissolve in water to give the solution with the lowest pH?
A.
B.
C.
D.
Which is an f-block element?
A. Sc
B. Sm
C. Sn
D. Sr
Which element is in the p-block?
A. Pb
B. Pm
C. Pt
D. Pu
Which metal has the strongest metallic bond?
A. Li
B. Na
C. K
D. Rb
Three elements, X, Y, and Z are in the same period of the periodic table. The relative sizes of their atoms are represented by the diagram.
Which general trends are correct?
Which oxide, when added to water, produces the solution with the highest pH?
A. Na2O
B. SO3
C. MgO
D. CO2
Which trends are correct across period 3 (from Na to Cl)?
I. Atomic radius decreases
II. Melting point increases
III. First ionization energy increases
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which equation represents the first electron affinity of chlorine?
A. Cl(g)+e-→ Cl-(g)
B. Cl2(g) + e- → Cl-(g)
C. Cl+(g) + e- → Cl(g)
D. Cl(g) → Cl+(g) + e-
Which property shows a general increase from left to right across period 2, Li to F?
A. Melting point
B. Electronegativity
C. Ionic radius
D. Electrical conductivity
Which element is a lanthanide?
A. Hf
B. Tb
C. U
D. Y
Which of the following shows a general increase across period 3 from to ?
A. Ionic radius
B. Atomic radius
C. Ionization energy
D. Melting point
Which describes the oxide of sodium, Na2O?
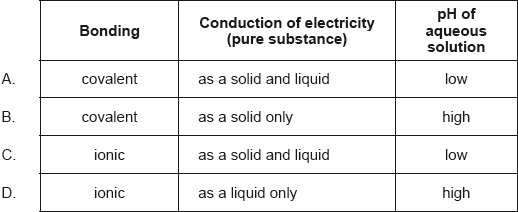
Which combination describes the acid–base nature of aluminium and phosphorus oxides?
Which species will require the least energy for the removal of one electron?
A. Na+
B. Mg+
C. Al2+
D. C3+
Which gases are acidic?
I. nitrogen dioxide
II. carbon dioxide
III. sulfur dioxide
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What are typical characteristics of metals?
Which of the following is the electron configuration of a metallic element?
A. [Ne] 3s2 3p2
B. [Ne] 3s2 3p4
C. [Ne] 3s2 3p6 3d3 4s2
D. [Ne] 3s2 3p6 3d10 4s2 4p5
Which oxide dissolves in water to give a solution with a pH below 7?
A. MgO
B. Li2O
C. CaO
D. P4O10
Which property increases down group 1?
A. atomic radius
B. electronegativity
C. first ionization energy
D. melting point
Which statement is correct?
A. Atomic radius decreases down group 17.
B. First ionization energy decreases down group 1.
C. Atomic radius increases across period 3 from Na to Cl.
D. First ionization energy decreases across period 3 from Na to Cl.
How do the following properties change down Group 17 of the periodic table?
Which oxides produce an acidic solution when added to water?
I. Al2O3 and SiO2
II. P4O6 and P4O10
III. NO2 and SO2
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which element has the highest metallic character in Group 14?
A. C
B. Si
C. Ge
D. Sn
Which element is found in the 4th group, 6th period of the periodic table?
A. Selenium
B. Lead
C. Chromium
D. Hafnium
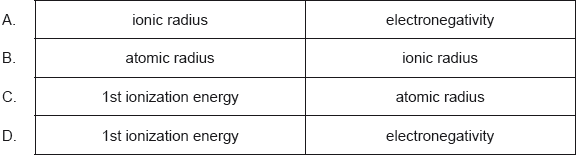
Which increase across a period from left to right?